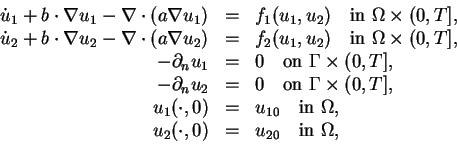 |
(1) |
Today, we will solve a system of convection-diffusion-reaction equations,
The
![]() formulation is given by
formulation is given by
Before today's computer session, make sure that you understand and can answer the following questions.
Question 1 Derive the variational formulation (2) from the system of equations (1).
Question 2
How does the corresponding variational formulation look for the
![]() method?
This method is also known as the Crank-Nicolson method.
method?
This method is also known as the Crank-Nicolson method.
Question 3
Assume that we have an (irreversible) chemical reaction of the form
![]() , let
, let ![]() be the concentration of the substance
be the concentration of the substance ![]() and let
and let
![]() be the concentration of the substance
be the concentration of the substance ![]() . Can you motivate
that this is modeled by the system (1) if we take
. Can you motivate
that this is modeled by the system (1) if we take
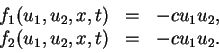 |
(3) |